Pharmaceutical and Biotech
(analytical chemistry development, CMC, and Formulation for Drug Product Development)
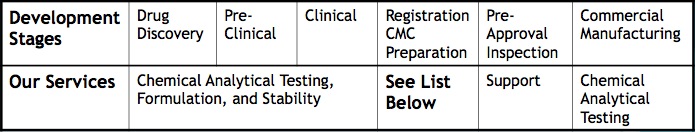
- Analytical method development and validation
- Analytical chemistry testing and forced degradation study for Active Pharmaceutical Ingredients (APIs) and finished products
- Stability studies supporting formulation feasibility, preclinical, and clinical studies
- Raw material (e.g.: APIs and excipients) qualification
- Formulation development for semi-solid and liquid dosage forms (e.g.: jelly, 70% Dex and injectables)
- API and drug product specification development
- Drug compatibility studies with excipients and packaging/closure materials
